BinaxNOW COVID-19 Ag Card is also a rapid test. Up to 7 cash back BinaxNOW COVID-19 Antigen Self Test 2 Tests.

Texas Math Sign Language Dictionary Math Signs Language Dictionary Sign Language Dictionary
The portable rapid molecular ID NOW COVID-19 test has emerged as a critical part of this arsenal allowing fast diagnosis with results in 13 minutes or less in a variety of locations such as physicians offices urgent care clinics and other point-of-care locations.

Abbott covid rapid test. BinaxNOW COVID-19 Ag Card Product Expiry Update May 2021 Dear Valued Customer. This test has received FDA Emergency Use Authorization for self-testing without the need to ship samples to a lab or for a prescription from your healthcare provider. Most rapid tests are antigen.
Food and Drug Administration FDA issued an Emergency Use Authorization EUA for. Food and Drug Administration FDA. Abbotts Binax COVID-19 Ag Card Abbot Laboratories Just as controversial new recommendations on.
This 15-minute test can be completed anytime anywhere. A simple solution for COVID-19 infection detection with rapid results in the convenience of your home. Cardinal Health will use its distribution network to support access to Quidels QuickVue At-Home OTC COVID-19 test and Abbotts BinaxNow COVID-19 antigen self-test.
This test offers results in 15 minutes and is available as an aid to diagnose the virus that causes COVID-19. What is the Abbott rapid COVID-19 test. Cardinal Health today announced that it has partnered with Abbott and Quidel to broaden access to over-the-counter rapid COVID-19 tests.
Its something that you can pick up at a store and do in the comfort of your home for yourself or even for your children. ID NOW COVID-19 is a rapid 13 minutes or less instrument-based isothermal test for the qualitative detection and diagnosis of -CoV-SARS2 from nasal nasopharyngeal and throat swabs. The tests can be used in point-of-care settings and at home with an online service provided by eMed.
A simple solution for COVID-19 infection detection with rapid results in the convenience of your home. Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start. Find out more about this innovative technology and its impact here.
Since the launch of the BinaxNOW COVID-19 Ag Card Abbott has continued testing for product stability to extend the expiration date and have shared these results with the FDA. Testing has been completed to support a shelf-life expiration date of up to 12 months. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less.
Rapid COVID-19 tests often provide results within minutes and dont need to be analyzed in a laboratory by a specialist. The test is a very simple to use tool that is allowing us to have more confidence as we come back to a new sense of normal in travel especially in everyday life. Abbott Laboratories announced on August 26 that the US.
The 5 COVID-19 test pairs with an app for a digital health pass after negative results. It is used on our ID NOW platform. Cardinal Health is partnering with Abbott and Quidel to commercialize and broaden access to over-the-counter rapid COVID-19 tests.
Results from the simple nasal swab are available in 15 minutes through testing individuals suspected of COVID-19. Abbott received emergency use authorization EUA from the US. This test has received FDA Emergency Use Authorization for self-testing without the need to ship samples to a lab or for a prescription from your healthcare provider.
It can be used at home by healthcare professionals at the point-of-care and or at home using a virtually guided service for the detection of antigens that form in the early stage of active infections. Food and Drug Administration FDA for the ID NOW COVID-19 test in March 2020. Cue our sixth COVID-19 test the BinaxNOW COVID-19 Ag Card rapid antigen test which has received emergency use authorization EUA from the US.

Teravista Is Proud To Be Located In Two Outstanding School Districts Georgetown And Round Rock O George Washington Carver Elementary Schools School District

As At Home Coronavirus Tests Hit Pharmacies What Role Can They Play In The Pandemic Npr
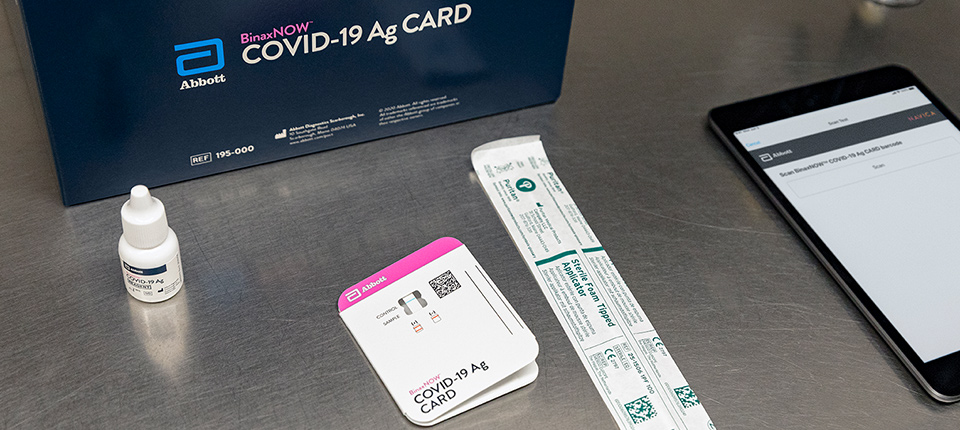
Covid 19 Innovation At Tech Conference Newsroom

The White House Bet On Abbott S Rapid Tests It Didn T Work Out The New York Times Compare Risk Communication Medicine Journal New York Times Preventive Measure

Genprice Inc In 2021 Preventive Healthcare Scientific Breakthroughs Scientific Journal

Abbott S 5 Covid 19 Rapid Test Wins Fda Emergency Use Nod 2020 08 27 Bioworld

دانيال براين يكشف السبب الغريب وراء رغبته في تحدي ذا روك سبورت 360 كشف نجم اتحاد Wwe لمصارعة المحترفين عن سبب غريب و Shane Mcmahon Wrestling Videos Wwe News

Our Quick Guide To Rapid Covid 19 Testing Abbott Newsroom

Abbott S Covid 19 Antigen Test Receives Ce Mark For Use On Children
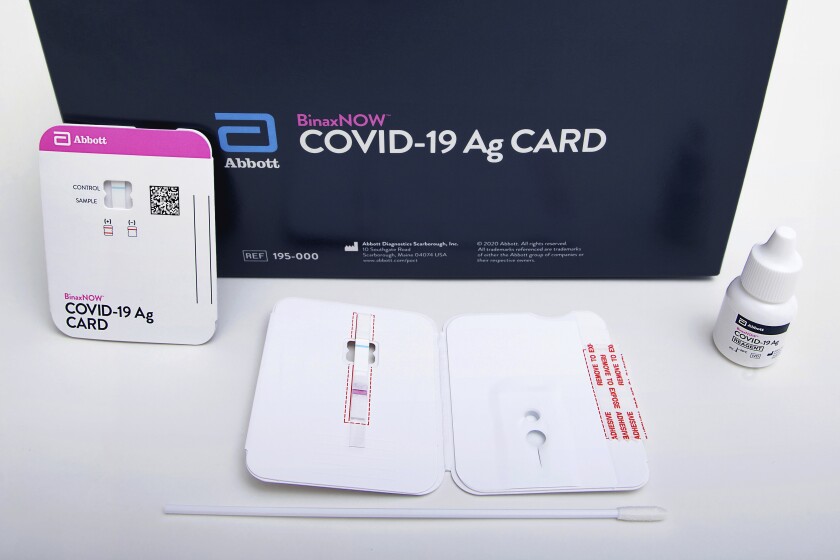
Fda Authorizes Covid 19 Test That Doesn T Need Special Equipment Los Angeles Times

Dynamic Pharma Medical Equipment Supplies In 2021 Medical Equipment Pharma Medical

Covid 19 Rapid Test By Abbott Labs Given Emergency Fda Approval Claims Accurate Results In 15 Minutes Abc7 Chicago

Dynamic Pharma Medical Equipment Supplies In 2021 Medical Equipment Pharma Medical

The Varioskan Lux Multimode Reader Simplifies Microplate Reading With Automatic Dynamic Range Selection Smart Safety Contro Smart Safety Thermo Fisher Readers

Dynamic Pharma Medical Equipment Supplies In 2021 Medical Equipment Pharma Medical



